
The order is summarized under the diagram.įIGURE 5.9 The arrow shows a second way of remembering the order in which sublevels fill.Īn atom of hydrogen (atomic number 1) has one proton and one electron. To use this figure, read along the diagonal lines in the direction of the arrow. The principal energy levels are listed in columns, starting at the left with the 1s level. This order is difficult to remember and often hard to determine from energy-level diagrams such as Figure 5.8Ī more convenient way to remember the order is to use Figure 5.9.

The first sublevel filled will be the 1s sublevel, then the 2s sublevel, the 2p sublevel, the 3s, 3p, 4s, 3d, and so on. Each added electron is assigned to the lowest-energy sublevel available. To determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of electrons equals the number of protons in the nucleus. As these molecules move downwards, they are pulled around and under the helium balloon, which pushes it upwards in reaction.The electron configuration of an atom shows the number of electrons in each sublevel in each energy level of the ground-state atom. It is because the air molecules around the helium balloon are heavier, and they are therefore pulled downward by gravity more strongly than helium. The reason that a helium balloon is buoyant in the atmosphere is not because it somehow generates an upward force. Helium will never ignite because, due to its di-electron stability, it is not interested in reacting with oxygen (or any other atom). Since hydrogen gas is so highly flammable, however, safety requires that helium be the gas of choice for buoyancy.
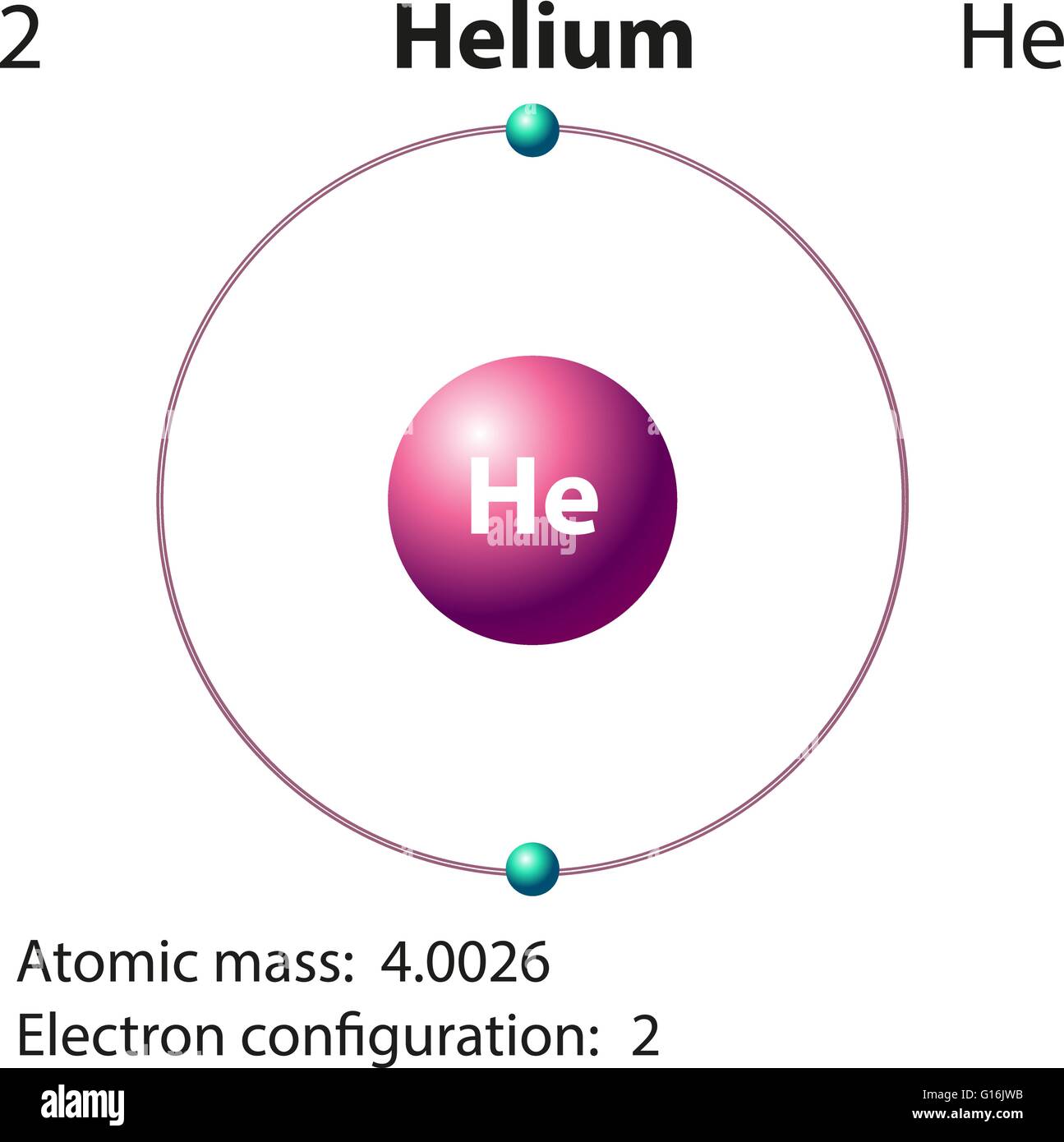
This makes them the best gases to use for buoyancy - in balloons and blimps - in the atmosphere. Since their atoms have the lowest masses, their gases have the lowest densities. Hydrogen (H 2) and helium (He) are the two lightest gases. With two proton-neutron pairs, the helium nucleus is itself a di-boson, making the helium atom a very stable tri-boson state. Alpha particle structure according to the Robinson Model of Nuclear Binding Most other nuclei are composed of various combinations of alpha particles ( see the Robinson Model of Nuclear Binding), which is why this is the only type of multi-nucleon structure that is ejected during radioactive decay.

Helium also contains the most stable nucleus - the alpha particle. Similar to the di-electron that envelops and binds the hydrogen molecule (H 2), helium’s two electrons form a very stable and perfectly spherical di-electron state - a boson state - where the two electron wave functions are completely superimposed upon one another. Since helium’s electrons are the most closely bound to their nucleus, helium has the highest ionization energy (2,370 kJ/mol or 24.5 eV) and is consequently the most unreactive element on the periodic table. This higher “effective nuclear charge” shrinks the atom’s size. It is smaller than hydrogen because it has twice as many protons in the nucleus attracting twice as many electrons inwards. Helium is the smallest atom on the periodic table. If the electron cloud were the size of a large football stadium, the nucleus would be the size of a penny at the center of the field - barely visible The size of the nucleus in the center is greatly exaggerated.


 0 kommentar(er)
0 kommentar(er)
